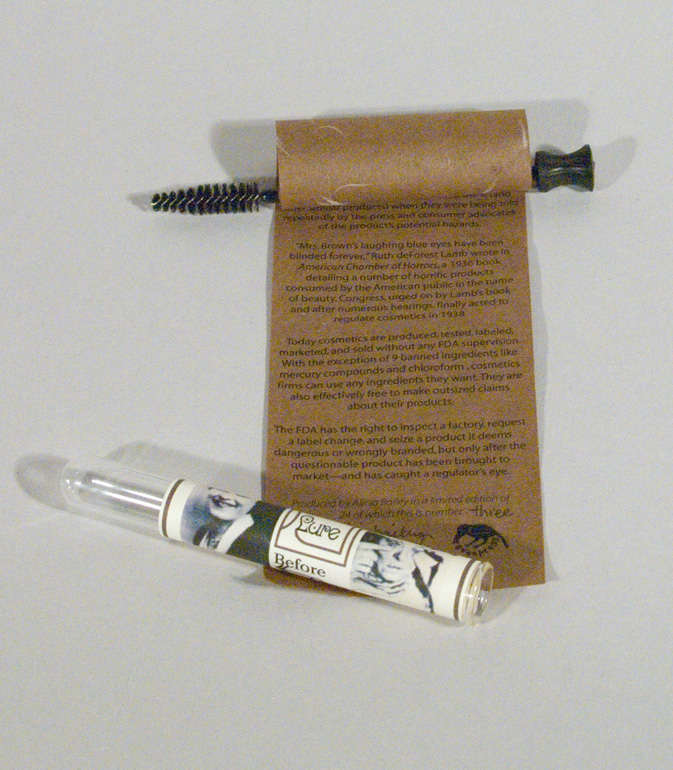
Lash Lure was the product that prompted FDA involvement with cosmetics in the 1930’s. This scroll book, housed in a test tube and wrapped around a mascara brush, tells the Lash Lure story.
On the morning of May 17, 1933, a woman known as Mrs. Brown stopped by Byrd’s Beauty Shoppe in Dayton, Ohio where she was encouraged to try a popular eyelash dye. The procedure was lengthy and messy. Almost immediately her eyes itched and burned. She applied a variety of ointments (boric acid, a concoction made up by her druggist followed by yellow oxide of mercury) in the hopes of alleviating her discomfort. The New Republic described her terrible morning after: “Her eyes are gone and the flesh around them is a mass of tortured scars.”
Today cosmetics are once again produced, tested, labeled, marketed, and sold without any FDA supervision. With the exception of nine banned ingredients like mercury compounds and chloroform and a list of color additives, cosmetics firms can use any ingredients they want. They are also effectively free to make outsized claims about their products. The FDA has the right to inspect a factory, request a label change, and seize a product it deems dangerous or wrongly branded, but only after the questionable product has been brought to market—and has caught a regulator’s eye.
Laserprint on Tairei paper mounted on mascara wand with wood bead handle house in glass test tube with color inkjet label.
Lash Lure – 2006
$ 75.00
c. 2006
edition of 24
4.5 x .5 x .5
In stock